How to Manage
Controlled Drugs
SageData is based in Ottawa, Ontario, Canada
Tracking Controlled Drugs
There are many regulations covering the issue and use of controlled drugs, and one of the
requirements relates to record keeping. This can be an onerous task. SDS (SageData) have
developed a system that shows how to manage controlled drugs and provide detailed reporting so
that proof of compliance can be provided when required.
SageData supplies the products and services you need to automate data collection and processing,
providing you with greater productivity, data accuracy, and insight into the operation of
your business.
For the Paramedic "business", data accuracy and insight into use of controlled medications isn't just desirable, it is mandated. BassetPro-Medic ensures that each individual vial is tracked from its first appearance under the Paramedic Service's control, to when it is used, or disposed. And other critical items can also be verified - vehicle keys, sat phones, emergency medical devices, trauma packs, fire extinguisher, etc., etc.
Features include:
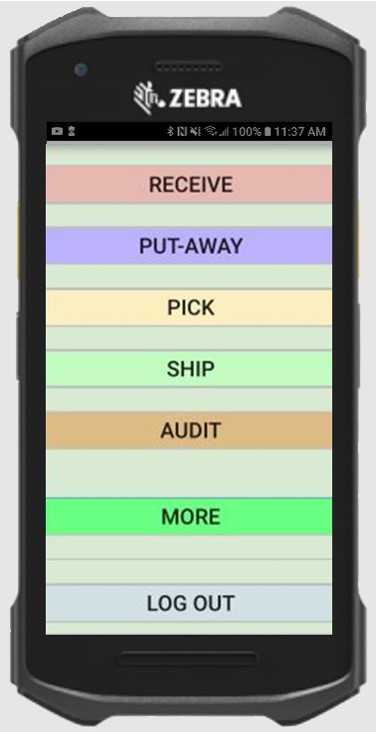 - Full lifecycle tracking of vials
- Full lifecycle tracking of vials
- Auto-ID scanning to ensure accuracy and speed
- Every inspection timestamped
- Personal vial responsibility tracked
- Mobile, touchscreen computing
- Easy to learn in minutes - intuitive buttons and instructions
- Easy to use - speeds up any current manual process
- Disposals tracked
- Tracking of other critical items
- Real-time information availability to paramedic managers across region
- Comprehensive reports available for audit verification
- Detailed history and management data analysis
We would be happy to shed more light on this important matter.
If you found this useful, you might also want to review:
-
an introduction to barcode technology
- an introduction to RFID
- mobile data collectors
-
consulting
services: barcodes and their applications
QAOK5358
